

Bohr also assumed that electrons orbiting the nucleus normally do not emit or absorb electromagnetic radiation, but do so when the electron switches to a different orbit. The Bohr model assumes that the electrons move in circular orbits that have quantized energies, angular momentum, and radii that are specified by a single quantum number, n = 1, 2, 3, …, but this quantization is an ad hoc assumption made by Bohr to incorporate quantization into an essentially classical mechanics description of the atom. Because of the quantized orbits, such “quantum jumps” will produce discrete spectra, in agreement with observations.īoth models have a central positively charged nucleus with electrons moving about the nucleus in accordance with the Coulomb electrostatic potential. The orbiting electron in Bohr’s model is assumed not to emit any electromagnetic radiation while moving about the nucleus in its stationary orbits, but the atom can emit or absorb electromagnetic radiation when the electron changes from one orbit to another. The Bohr model retains the classical mechanics view of circular orbits confined to planes having constant energy and angular momentum, but restricts these to quantized values dependent on a single quantum number, n. If classical electromagnetic theory is applied, then the Rutherford atom would emit electromagnetic radiation of continually increasing frequency (contrary to the observed discrete spectra), thereby losing energy until the atom collapsed in an absurdly short time (contrary to the observed long-term stability of atoms). If the requirements of classical electromagnetic theory that electrons in such orbits would emit electromagnetic radiation are ignored, such atoms would be stable, having constant energy and angular momentum, but would not emit any visible light (contrary to observation).

According to classical mechanics, the Rutherford model predicts a miniature “solar system” with electrons moving about the nucleus in circular or elliptical orbits that are confined to planes. Take advantage of the fast search and powerful cloud editor to produce an accurate Chapter 4 Atomic Structure Wordwise Answer Key.Both involve a relatively heavy nucleus with electrons moving around it, although strictly speaking, the Bohr model works only for one-electron atoms or ions.
#CHAPTER 4 ATOMIC STRUCTURE ANSWER KEY DOWNLOAD#
Download the document or print out your copy.
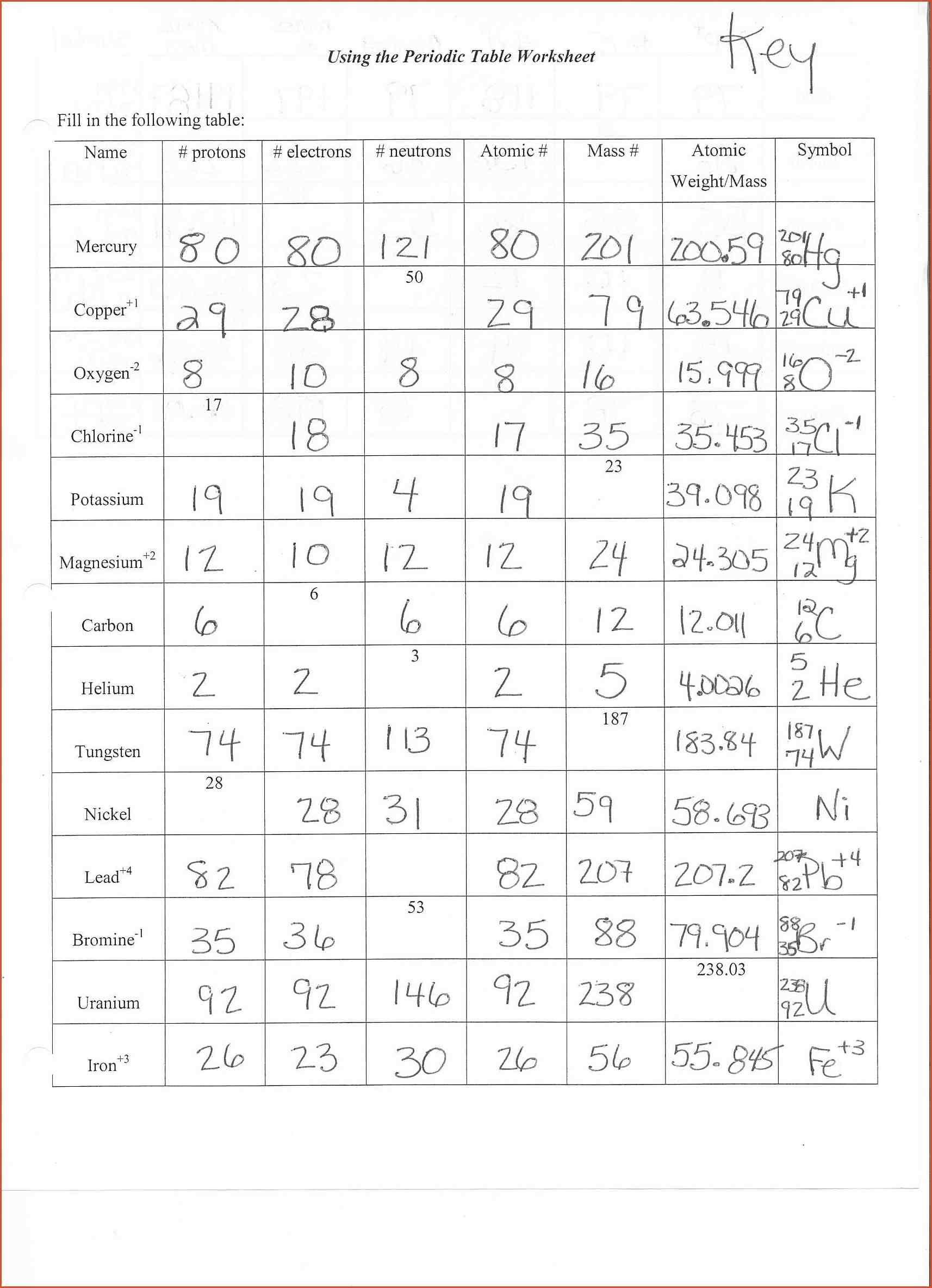
Check if everything is filled in appropriately, without any typos or missing blocks.The intuitive drag&drop interface makes it simple to add or move areas.
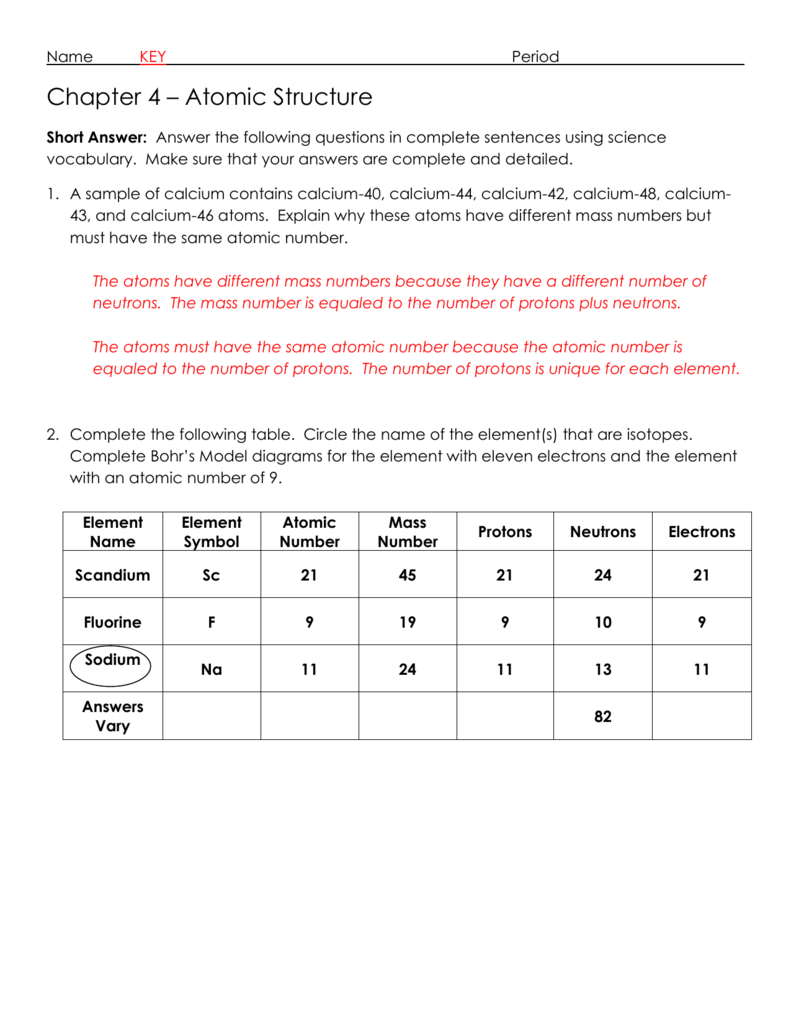
Type all required information in the required fillable fields.Our state web-based samples and simple recommendations eradicate human-prone mistakes.Ĭomply with our easy steps to have your Chapter 4 Atomic Structure Wordwise Answer Key prepared quickly: Now, using a Chapter 4 Atomic Structure Wordwise Answer Key requires no more than 5 minutes. However, with our predesigned online templates, things get simpler. The preparation of lawful paperwork can be high-priced and time-consuming.


 0 kommentar(er)
0 kommentar(er)
